How to Remove Hardness from Water at Home
- by Ryan Moreau / updated April 23rd, 2024
Hard water is a common problem faced by many households in the United States. The primary concern with hard water is scale deposits that form on plumbing and appliances. These deposits start to build up over time and can reduce water flow and cause significant damage. Hard water can also have a negative impact on skin and hair health.
The issues associated with hard water are often misunderstood, in large part due to misinformation from companies that sell water softeners. The presence of hardness in your water does not necessarily mean you will have issues with hard water deposits. Several factors influence water’s potential to form hardness deposits.
In this guide, we will discuss water hardness in greater detail and help you determine if hard water is an issue in your household.

Contents
What is Hardness?
Hardness is made up of dissolved calcium and magnesium ions. The total hardness in your water is the sum of both of these dissolved minerals. These minerals are naturally occurring and can be found in varying levels in water sources worldwide. High hardness is typically found in water that comes from a well or groundwater source. This is also highly dependent on geography. For example, if you own a well in the Midwest, you will likely have very high hardness levels. However, if you own a well on the East Coast, the hardness levels will be extremely low.
There are disadvantages and advantages to both high and low hardness, and typically, the best water is somewhere in the middle with moderate levels of hardness. Very high hardness will lead to deposits in your plumbing and appliances, and very low hardness can lead to excessive corrosion of pipes throughout your house.
There are two main types of hardness: temporary and permanent. Temporary hardness is also known as carbonate hardness, which can be removed by boiling water. Permanent hardness, on the other hand, is non-carbonate hardness, and it cannot be removed by boiling due to greater solubility in water.
Boiling works to remove carbonate-based hardness because calcium carbonate is inversely soluble. This means that the hotter the water, the less likely hardness is to stay dissolved in water. As the water is heated up by boiling, calcium carbonate deposits will start to form in the water and on surfaces. This is why hardness deposits are mostly found in the hottest area of your home, such as your water heater, shower, and dishwasher.
General guidelines for classification of waters are 0 to 60 mg/L (milligrams per liter) as calcium carbonate is classified as soft; 61 to 120 mg/L as moderately hard; 121 to 180 mg/L as hard; and more than 180 mg/L as very hard. Water with low deposit and low corrosion potential will typically fall in the moderately hard range.
The Negative Effects of Hard Water
Hard Water Deposits
Hard water deposits can cause significant damage to home plumbing and appliances such as hot water heaters, shower heads, and washing machines. The buildup of minerals in these appliances can also reduce their efficiency and lifespan and lead to costly maintenance, increased utility costs, and costly replacements.
Hardness deposit formation is more than just a function of the hardness level in your water. It’s also highly dependent on your water’s pH, alkalinity, TDS, and temperature. The potential for water to form hardness scale can be determined using the Langliers Saturation Index (LSI). You can easily test these parameters yourself and enter the values into the LSI calculator below to determine if your water is deposit-forming or corrosive.
Langliers Saturation Index
Deposit and Corrosion Calculator
If the results of your water show high to very high deposit potential, you should consider installing a softener.
Signs that you may need a softener include:
- Water spots and stains on shower and dishes
- Dry hair and skin
- Difficulty getting laundry clean
- Poor hot water flow and visible signs of hardness scale on household plumbing and appliances
Ignoring these concerns can lead to premature failure of your water heating, plumbing, and other household appliances.
If your water shows moderate to very high corrosion potential, you should consider installing an acid neutralizer.
Skin and Hair Health
The effects of hard water on skin and hair can be a nuisance for some people. Hard water can cause dry, itchy skin and lead to, irritation, redness, and even exacerbate conditions like eczema and acne. In the case of hair, hard water can make it dry, brittle, and dull, while also causing scalp issues and fading hair color.
Outside of installing a water softener, these issues can be countered using gentle skincare and haircare products, rinsing with distilled water, moisturizing, and maintaining clean fixtures to reduce mineral buildup. The specific impact may vary depending on individual sensitivity and water mineral content, but these strategies can help keep skin and hair healthy in hard water areas.
Testing for Water Hardness
Local Water Quality Report
The easiest method to get the approximate water hardness levels in your water is to look up your city or well water using our Taptool water quality report. After you enter your zip code and either city or well water, your water report will return the average local hardness levels as well as the additional parameters needed to calculate the deposit or corrosion potential of your water. Below is an example of what your report might look like.
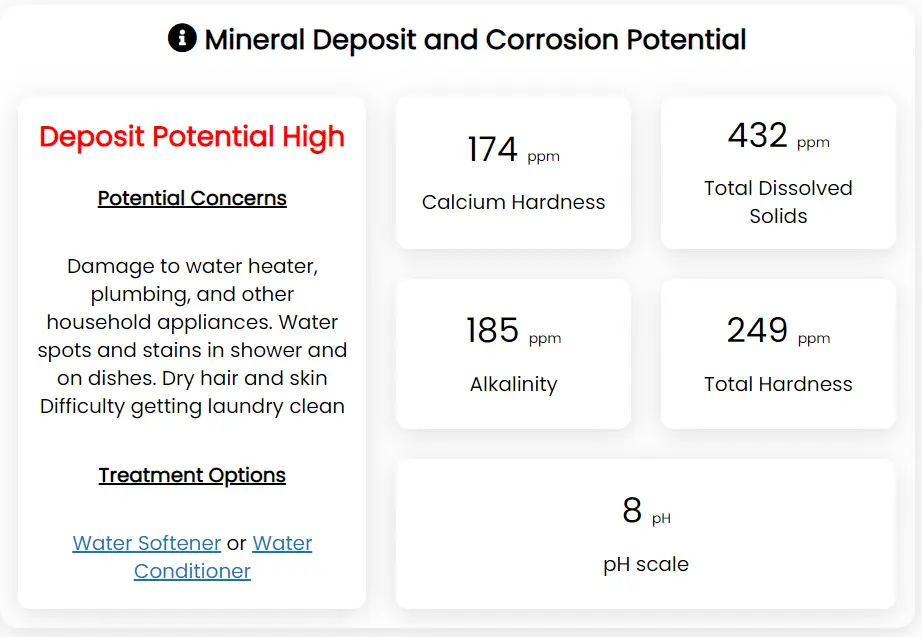
The data used in our taptool report from this section is average local source water data from the United States Geological Survey (USGS). In most cases, this is very accurate based on local geology and gives a good approximation of what you can expect at your tap. However, in some cases, your local water supply may have treatment in place to reduce the total hardness level. This is most often done using precipitation treatment such as cold lime softening. In this process, calcium hydroxide (lime) and sodium carbonate are added to the water to precipitate hardness ions as an insoluble precipitate that is removed in a clarifier. If your public utility softens the water, testing your water at the tap will give you more accurate hardness levels.
Hardness Test Kits
The best way to determine the hardness levels in your water is to test it at your tap. Testing the hardness of your water at home can be done using cheap and accurate at-home test kits. Since most hardness deposits are composed of calcium carbonate, it is important to test the calcium portion of hardness to calculate the potential of deposits to form. Testing just the calcium hardness does not tell the whole story, you will also need to test the pH, alkalinity, and TDS of your water. Once you have all these values, you can plug them into our LSI calculator to determine the potential for hardness scale to form in your house. For more detailed info on water testing, check out our water testing guide.
How to Remove Hardness from Water
Hard water is best treated using one of two treatment methods: either a water softener or a water conditioner. These systems are best installed at the point of entry to the house so that hardness is removed before reaching any plumbing or appliances. Since hardness mostly deposits in the hottest areas, some people soften only the hot water side.
Water Softener
Water softening is a common method used to remove hardness from water. Home water softeners work via an ion exchange process, exchanging calcium and magnesium ions in the water with sodium ions. The softener vessel contains a resin bed with an affinity for cations such as sodium, calcium, and magnesium. Hardness removal is achieved through an equilibrium reaction as the ion exchange resin prefers the hardness ions at a higher concentration, releasing sodium ions in favor of the hardness ions. Once the resin beads are fully saturated with hardness ions, the bed is regenerated using sodium chloride (salt), shifting the equilibrium back to sodium and resulting in the removal of hardness from the resin beads.
For most people, the amount of sodium added to water by a water softener is relatively small and not a significant concern. However, for individuals on a sodium-restricted diet, every source of sodium in their diet needs to be carefully monitored. Even small amounts of additional sodium from softened water can contribute to an increase in daily sodium intake, which can be problematic for those with health risks like high blood pressure, cardiovascular disease, or kidney problems.

Water Conditioner
Another treatment option for hard water is a salt-free water softener or water conditioner. Water conditioners do not actually make soft water; instead, the water hardness minerals are altered through template-assisted crystallization. These altered minerals do not form deposits on surfaces within your home. In this process, water flows through a bed of polymeric beads, turning dissolved hardness into microscopic crystals that remain in solution without forming scale deposits in your home. The advantage of this process over traditional softening is that no regeneration is needed. Because of this, the water consumption of a water conditioner is much less, and you do not have to use salt or connect the unit to a drain.
In some areas of the US, water softeners are not allowed due to high chloride content water discharge from the regeneration and backwash step. Typically, these areas experience drought, and they regulate water softeners to prevent contamination of their water source with this high chloride content water wasted during the regeneration process. Unlike softening, this process does not remove calcium and magnesium from your water, which is an additional health benefit.
One disadvantage of no-salt systems is they’re not as effective in places where water sits, like in your water heater. Another issue is that saltless water conditioners don’t work equally well in all conditions or for all systems. For instance, salt-free conditioners generally should not be used with well water. The types of contaminants you have in your water, which can vary widely from one municipality to another, can affect the operation of your system
If you have any further questions about hardness in water, please feel free to Contact us.
